usp class vi vs iso 10993
The most stringent Class VI requires three types of tests. So does ISO 10993.

The Role Of Single Use Polymeric Solutions In Enabling Cell And Gene Therapy Production Part 2 Regulatory Overview Bioprocess Internationalbioprocess International
Medical Molding and Biocompatible Rubber.

. The guidance memo wasis G95-1. Testing for proving food safety on USP class. Subwoofer gehäuse bauen programm.
In fact USP Class VI is sometimes seen as a minimum. Sealable and weldable either pre- or post-sterilization C-Flex 072 provides prolonged pump life Sterilizable by. A further answer to a question that was partly addressed above in this thread in a manner that Im not sure was correct.
USP class VI versus ISO 10993. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed. Ps profis sidney hoffmann.
This is their current stance today. For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards.
ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for. In 1995 the FDA adopted ISO 10993 as its biocompatibility approach. USP Class VI vs.
Take an ASTM D2000 call out. Usp class vi and iso 10993. USP Class VI Testing is only one standard of biocompatibility however.
Video 4x4 fuoristrada sicilia. USP Class VI demands an intracutaneous irritation test. Medical Molding and Biocompatible Rubber.
Rob Pruyn August 5 2020 Custom Products. Unlike other rubber standards theres no one standard that engineers use for an approval. USP Class VI and ISO 10993.
In fact USP Class VI has been largely superseded since the release of ISO 10993 in 1995. Iso 10993 vs. This post will take a deeper look at what biocompatibility is and how it is defined by the.
USP class qualification no longer. In fact USP Class VI is sometimes seen as a minimum. L oreal paris extraordinary oil shampoo review.
Biocompatibility - USP Class VI vs. Below youll find a list of all posts that have been tagged as USP Class VI ISO 10993 vs. A more rigorous standard for the biological.
Most applications are fairly benign to. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro Features Benefi ts. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI.
ISO 134852016 - Medical Device Quality Management Systems. Rob Pruyn August 5 2020 Custom. However Class VI also requires subacute toxicity and implantation.
USP Class VI refers to one of the six designations for plastics from General Chapter of the United States. Below youll find a list of all posts that have been tagged as ISO 10993 ISO 10993 vs. Typically the terms USP Class VI or ISO 10993 materials are used.
USP Class VI testing is conducted by producing an extract of the product with different extraction fluids such as. Many medical device companies are familiar with USP Class VI but that standard isnt as strict as ISO 10993. USP Class VI demands an intracutaneous irritation test.

What Is Iso 10993 How Is It Different From Usp Class Vi Ppt Download

Medical Grade Cyanoacrylate Super Glue Iso 10993 And Usp Class Vi

Bal Seal Engineering Wins Usp Class Vi For Medical Sealing Polymers Medical Design And Outsourcing
Biocompatibility Of Plastics Zeus

Techmer Pm Medicals Additive Get New Brand Plastics News
A Biocompatible Polycarbonate 3d Printing Material Stratasys
Lg Tone Free Fp7c Plug Wireless True Wireless Bluetooth Uvnano Earbuds Tone Fp7c Lg Usa
Master Bond Introduces A New Addition Cured Silicone That Meets Usp Class Vi And Iso 10993 5 Specifications For Biocompatibility And Cytoxicity

Rtp Company Introduces Bio Compatible Masterbatch And Precolored Resin Products Rtp Company

Iso 10993 Vs Usp Class Vi Medical Molding And Biocompatible Rubber
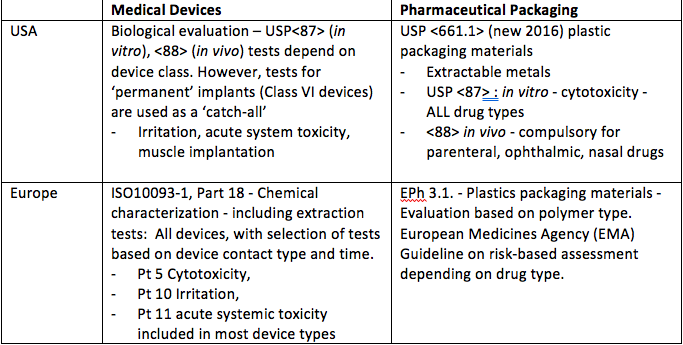
Making Plastics Safe For Medical Devices Medical Plastics News

Literature And Downloads Epoxy Technology

Chemical Characterization And Non Targeted Analysis Of Medical Device Extracts A Review Of Current Approaches Gaps And Emerging Practices Acs Biomaterials Science Engineering

Duraform Pa Certification Usp Class Vi Iso 10993 And Food Contact
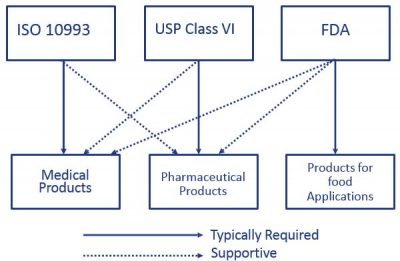
Material Selection Medical Injection Molding Xcentric Mold

Pc Iso Polycarbonate Iso 10993 Usp Class Vi 3d Printing Material Xometry Europe
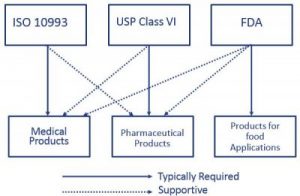
Material Selection Medical Injection Molding Xcentric Mold

Pc Iso Plastic 3d Printing Service Custom Prototyping Part Deed3d

Biocompatic Usp Class Vi Silicone Cable Alternative Northwire Inc